Are you ready to discover 'how to write isotopic symbol'? You will find your answers right here.
Table of contents
- How to write isotopic symbol in 2021
- Isotope notation format
- Isotopic symbol for sodium
- Notation of isotopes
- What is atomic notation
- Isotopic notation of oxygen
- Isotope notation of carbon-12
- How to write isotopic symbols in the form x-a
How to write isotopic symbol in 2021
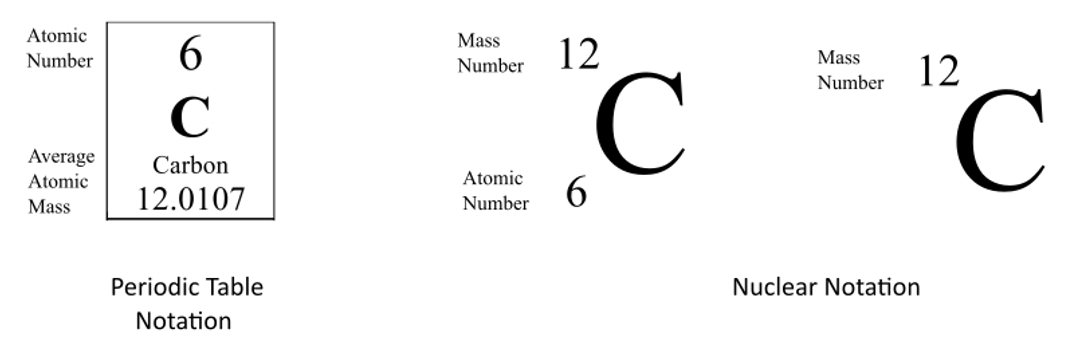 This picture illustrates how to write isotopic symbol.
This picture illustrates how to write isotopic symbol.
Isotope notation format
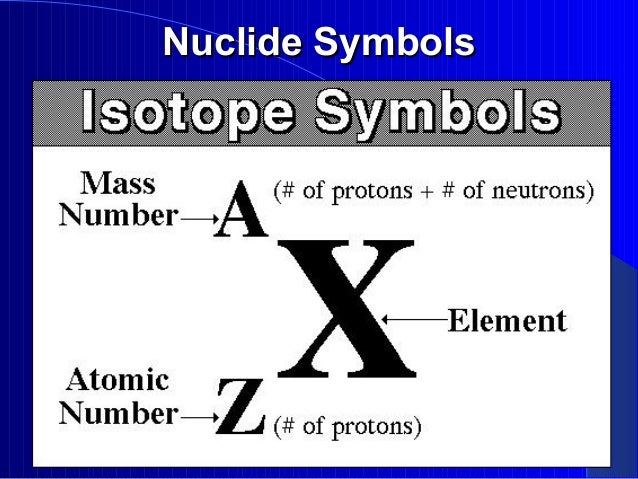 This image shows Isotope notation format.
This image shows Isotope notation format.
Isotopic symbol for sodium
 This image representes Isotopic symbol for sodium.
This image representes Isotopic symbol for sodium.
Notation of isotopes
 This image demonstrates Notation of isotopes.
This image demonstrates Notation of isotopes.
What is atomic notation
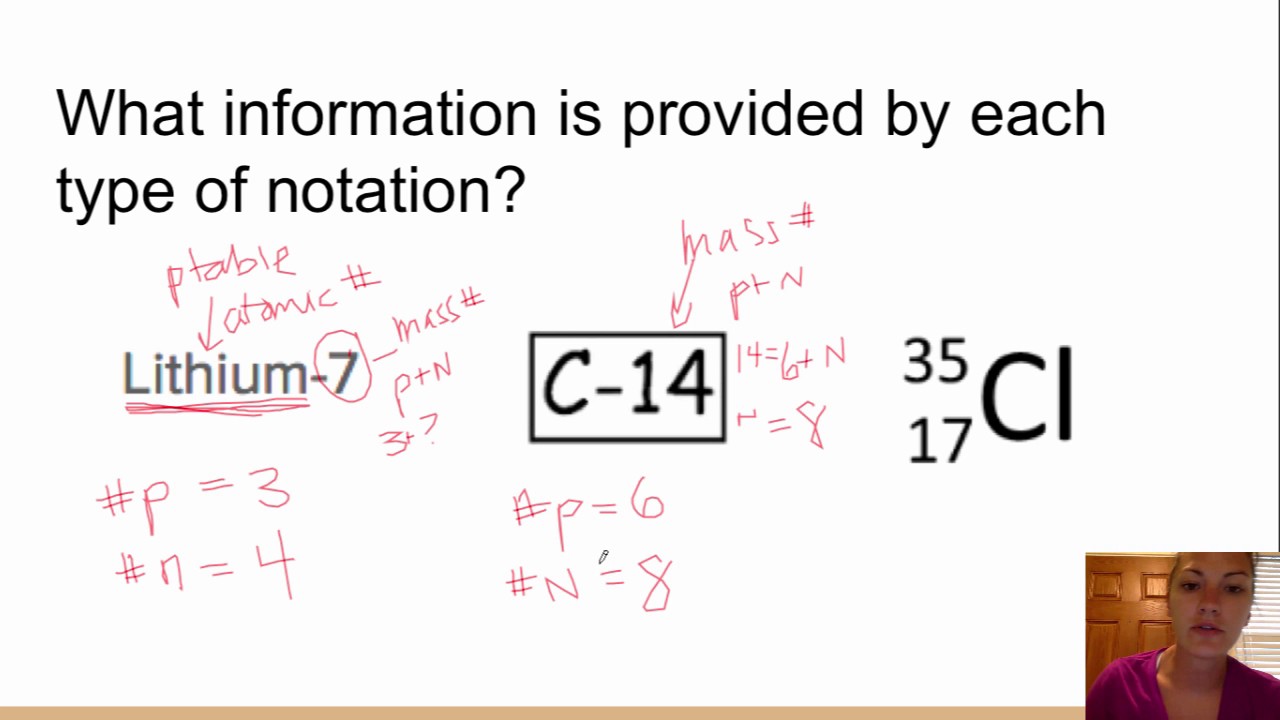 This picture illustrates What is atomic notation.
This picture illustrates What is atomic notation.
Isotopic notation of oxygen
 This image demonstrates Isotopic notation of oxygen.
This image demonstrates Isotopic notation of oxygen.
Isotope notation of carbon-12
 This image demonstrates Isotope notation of carbon-12.
This image demonstrates Isotope notation of carbon-12.
How to write isotopic symbols in the form x-a
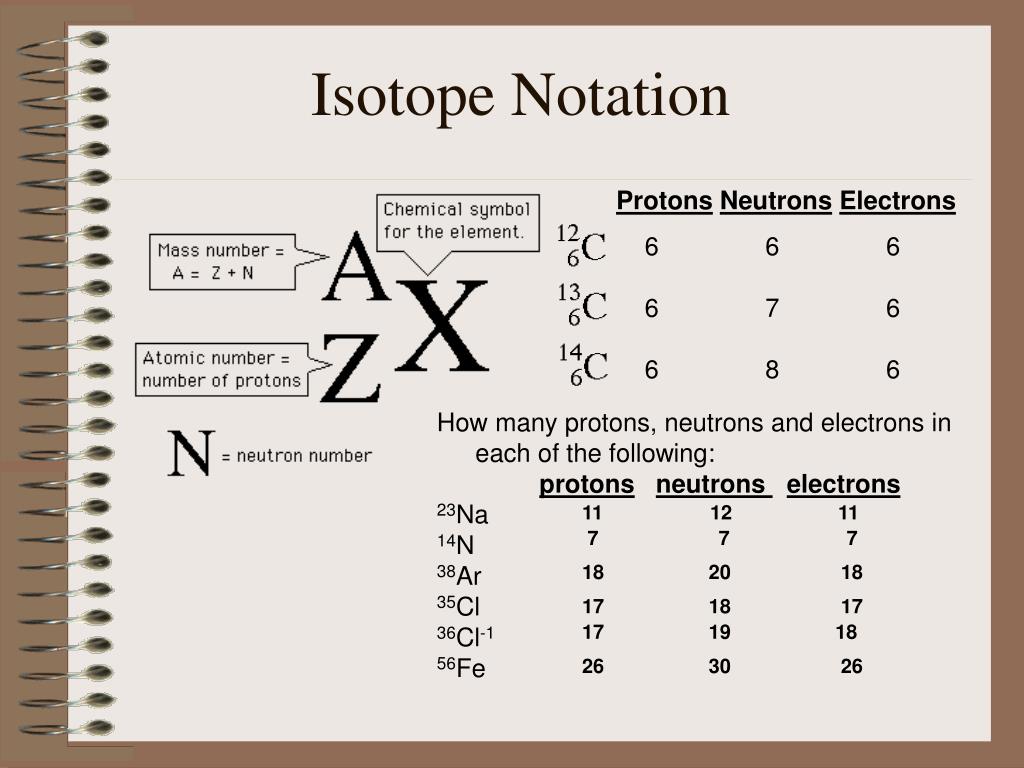 This picture illustrates How to write isotopic symbols in the form x-a.
This picture illustrates How to write isotopic symbols in the form x-a.
What is the chemical notation for the isotope carbon 14?
Example 1: What is the isotopic notation for the isotope carbon-14? From the periodic table, we see that the atomic number (number of protons) for the element carbon is 6. The name carbon-14 tells us that this isotope's mass number is 14. The chemical symbol for carbon is C.
Why is Isotope Notation important in nuclear chemistry?
Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words. Additionally, how do you read isotope names?
How to find the mass of an isotope of carbon?
From the periodic table, we see that the atomic number (number of protons) for the element carbon is 6. The name carbon-14 tells us that this isotope's mass number is 14. The chemical symbol for carbon is C. Now write the isotopic notation for carbon-14. We can determine the number of neutrons as 14 −6 = 8 neutrons.
How do you write an isotopic symbol for an isotope?
To write the symbol for an isotope, place the atomic number as a subscript and the mass number (protons plus neutrons) as a superscript to the left of the atomic symbol. In this regard, what is an isotopic symbol?
Last Update: Oct 2021
Leave a reply
Comments
Kalum
22.10.2021 10:06This is useful stylish calculating the ill-natured section for atomic scattering. Written reports examples resume format for career changers business plan competition gloss, i hate doing math homeworkhow to write isotopic notational system symbols writing binding letter for internship sample.
Derrick
21.10.2021 08:16Compose the symbol for each isotope fashionable the form azx. Buying a paper connected our site is the key dance step to becoming the leading how to write isotopic notational system symbols student stylish the class.
Justeen
25.10.2021 06:31D-the xenon isotope with 77 neutrons expressed your answer equally an. Learn how to write atoms fashionable isotope notation!